U-M BME researchers, including Lonnie Shea, Steven A Goldstein Collegiate Professor, Biomedical Engineering, have contributed to developing a strategy to enhance neurorepair through a temporally layered approach using acute bridge implantation and chronic cell transplantation to spare tissue, promote regeneration, and maximize the function of new axonal connections. University of California, Irvine led the research.
This study, published in Nature Regenerative Medicine, highlights regeneration in the injured spinal cord, which traditionally is limited by physical and chemical barriers. Acute implantation of a multichannel poly(lactide-co-glycolide) (PLG) bridge mechanically stabilizes the injury, modulates inflammation, and provides a permissive environment for rapid cellularization and robust axonal regrowth through this otherwise inhibitory milieu.
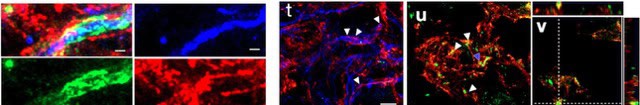
However, without additional intervention, functional repair is limited owing to the regenerated axons remaining largely unmyelinated (<10%). Similar to insulation, oligodendrocytes cells create a membranous sheath surrounding axons, called myelin. Myelin acts as an insulator that speeds electrical transmission. While transplanted human neural stem cells (hNSC) can develop into oligodendrocytes and myelinate axons after spinal cord injury (SCI), hNSC fate is highly influenced by the SCI inflammatory microenvironment, which the biomaterials can modulate.
This research was possible due to collaboration between an engineer (Shea) and neuroscientists Aileen Anderson, PhD and Brian Cummings, PhD, who have worked with neural stem cells for many years. Dr. Shea noted that “either approach alone is able to support only limited functional recovery; however, the combination has led to substantial improvements.”
The research team investigated the combination of PLG scaffold bridges with hNSC to improve histological and functional outcome after SCI. In vivo, acute PLG bridge implantation followed by chronic hNSC transplantation demonstrated a robust capacity of donor human cells to migrate into PLG bridge channels along regenerating axons and integrate into the host spinal cord as myelinating oligodendrocytes and synaptically integrated neurons. Axons that regenerated through the PLG bridge formed synaptic circuits that connected ipsilateral forelimb muscle to contralateral motor cortex. hNSC transplantation significantly enhanced the total number of regenerating and myelinated axons identified within the PLG bridge. Finally, the combination of acute bridge implantation and hNSC transplantation exhibited robust improvement in locomotor recovery.