U-M BME-led study reveals the key role of anti-tumor neutrophils in suppressing lung metastasis of breast cancer
The research team employed a subcutaneous biomaterial scaffold implant

The research team employed a subcutaneous biomaterial scaffold implant
A U-M BME-led study published in Nature Communications on August 8 reveals the multifaceted roles of neutrophils in regulating lung metastasis in breast cancer and underscores the key role of neutrophils with anti-tumor phenotypes in suppressing breast cancer cell growth in the lungs.
This study was led by Dr. Lonnie Shea, the U-M BME Steven A. Goldstein Collegiate Professor with appointments in surgery and chemical engineering, and Dr. Jacqueline Jeruss, U-M Associate Dean for Regulatory Affairs, Associate Vice President for Research, and professor of surgery, pathology and BME. Dr. Jing Wang, formerly a postdoctoral fellow at U-M BME and currently an assistant professor at Iowa State University, is the first author. Dr. Aaron Morris, U-M BME assistant professor, is a co-author on this work.
The research team employed a subcutaneous biomaterial scaffold implant to mimic the immune environment in metastatic organs and deconstruct complex signals regulating metastasis. The scaffold implant in mouse models of metastatic breast cancer creates a synthetic metastatic niche, an environment that can recruit lung-tropic circulating tumor cells (tumor cells that preferentially migrate to the lungs) yet suppress their growth through potent in situ anti-tumor immunity. In contrast, similar circulating tumor cells seed the lungs, the endogenous metastatic organ for these models, and develop into lethal metastatic tumors as the environment in the lungs becomes immunosuppressive and pro-tumor at this stage of breast cancer metastatic progression.
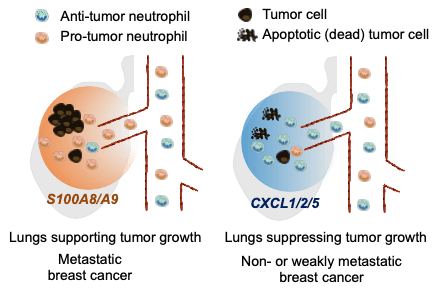
The team examined why scaffolds and lungs in the same mouse create different immune environments and direct opposite fates of metastatic breast cancer cells. They found that the selective recruitment of neutrophils with different phenotypes from the circulation determines that the overall environment in the scaffold or lungs is immune-stimulatory and anti-tumor or immunosuppressive and pro-tumor, respectively. “Our study indicates that the scaffold implants overproduce chemokines CXCL1, CXCL2, and CXCL5 due to the foreign body responses to scaffold implants.” Dr. Wang said. “And these chemokines recruit anti-tumor neutrophils from the circulation to activate tumor-killing effector cells (CD8+ T cells and NK cells) to clear tumor cells seeding the scaffolds. In contrast, the lungs in mice with metastatic breast cancer overproduce S100A8/A9 protein complex to recruit pro-tumor neutrophils that suppress the activity of effector cells and support tumor cell growth.” A scheme is provided in Figure 1.
Additionally, their findings from the synthetic metastatic niche further explain why the lungs from the host with non- or weakly metastatic breast cancers do not develop metastatic tumors. In contrast to the lungs in the setting of metastatic breast cancer that are dominated by pro-tumor neutrophils and deactivated effector cells, the lungs in non- or weakly metastatic breast cancers overproduce CXCL1, CXCL2, and CXCL5, possibly due to immunoediting of primary tumors, to recruit and enrich anti-tumor neutrophils and create an environment with potent anti-tumor immunity that prevents the growth of metastatic cells.
“Our findings have been validated and may have clinical implications. A high ratio of signals that recruit anti-tumor neutrophils (Cxcl1, Cxcl2, or Cxcl5 genes) to signals that recruit pro-tumor neutrophils (S100a8, or S100a9 genes) was positively correlated to a low chance of developing metastasis in our study. This ratio may help to predict the risk level of lung metastasis for patients with breast cancer.” said Dr. Wang. Dr. Shea added that the scaffolds are being developed for a clinical study, and this ratio could be derived from the scaffold, thereby providing an opportunity to assess metastatic disease and guide patient management without an invasive biopsy of vital organs.
Wang added: “Our findings also have therapeutic implications, as this mechanistic study will inspire new anti-metastasis therapy strategies directed towards changing the signals in the lungs from those recruiting pro-tumor neutrophils to those recruiting anti-tumor neutrophils.”

Figure 1. Schematic illustration of the selective recruitment of anti-tumor and pro-tumor neutrophils to the lungs of animal models of breast cancers with varying tumor aggressiveness responding to distinct groups of chemokines/cytokines that attract neutrophils, followed by creation of an environment in the lungs that supports or suppresses growth of breast cancer cells.