A recently published study in Acta Biomaterialia featuring the research of BME Ph.D. student Irene Zhang and Andrew Putnam, Ph.D., Robert C. Leland, Jr. and Donna D. Leland Professor, Biomedical Engineering and Cardiovascular Medicine, holds the promise of overcoming longstanding challenges in the field of tissue engineering by creating advanced vascular constructs that might one day pave the way for building larger, clinically relevant tissues and potentially whole organs.
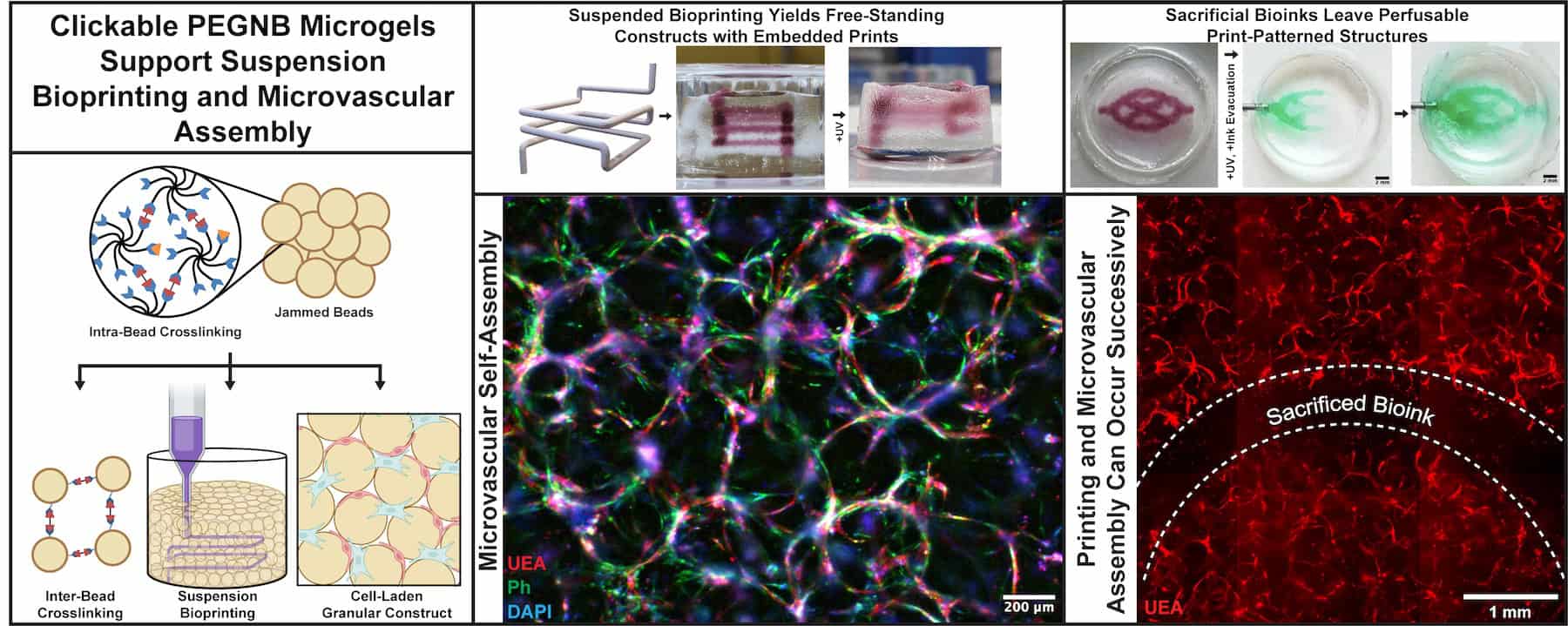
In this study, the team leveraged and combined advances in microgel biomaterials, granular hydrogels, suspension bioprinting, and vascular biology to create relatively large volume vascularized constructs. Other BME collaborators included Jan Stegemann, Ph.D., Professor, Biomedical Engineering; Brendon Baker, Ph.D., Associate Professor, Biomedical Engineering; and Sasha Cai Lesher-Perez, Assistant Professor, Chemical Engineering and Biomedical Engineering.
“The combination of top-down and bottom-up approaches within a single construct represents a significant and innovative contribution that we believe will be of broad interest to the biomaterials and regenerative medicine communities,” Zhang said.
The cornerstone of this research blended novel materials and bioprinting techniques to engineer vascular systems that could support the complex tissue structures necessary for transplantation and regenerative medicine. Dr. Putnam, who has dedicated more than two decades to researching vascularization, described the study’s significance: “Irene has really enabled a major advancement, and I think that’s what I’m most excited about. Now, we can go and hopefully soon talk to a clinician and show them something that’s getting pretty close to what they are asking for–a construct where you could suture vessels in place. That’s really the ultimate goal.”
The research employs a unique approach combining granular materials and suspension bath bioprinting to develop scalable, hierarchical vasculature that mimics the natural multi-scale structure found in human organs. “It’s about making a seamless way to form hierarchical vasculature,” Zhang explained. “We’re trying to show that we’re able to optimize these granular materials that can support embedded bioprinting and secondarily cross-link, forming a manipulatable, culturable construct where cells self-assemble into vasculature.”
The potential applications for these engineered vascular models are vast, ranging from skin grafts to components of major organs such as the liver or heart. Dr. Putnam noted, “In theory, it could be any tissue type or organ in the body. We think of this more as a platform technology–a tool that people can use.”
When asked about the next steps in research, Zhang was optimistic. “The integration of vascular structures produced by the combination of top-down and bottom-up approaches is the step I’m actively working on,” she said. “This also involves integrating more metabolically active cell types, such as muscle or cardiac cells, to see if they can be sustained within this new vascular framework.”
Zhang entered graduate school interested in bioprinting and tissue engineering, and her research focus has been supported through the Cellular Biotechnology Training Program, which required her to complete an industrial internship—a formative experience that strengthened her interest in pursuing applications in this field.
Dr. Putnam acknowledges that the research is still at a developmental stage, but is encouraged by its implications for the future. “Five to ten years is the short answer,” he said when asked about the arrival of potential clinical applications, adding, “If we could implement this in ischemic disease states, for example, in patients who have poor blood flow due to cardiovascular issues, that would be a more viable, quicker target.”
Zhang’s research is not only groundbreaking for its scientific merit, but also for its collaborative integration of different technological domains. “Combining those two elements, the printing and these granular materials, is what I think we’ve really contributed as an advance,” Dr. Putnam emphasized.
“I really want to highlight that to the best of our knowledge, this is the first study that uses poly(ethylene glycol) (PEG) microgels as supportive materials for bioprinting, and one of the first papers to document microvascular self-assembly within granular constructs to marry these features into one,” Dr. Putnam said.