Unlocking the Secrets of the Oral Microbiome To Prevent and Manage Dental Diseases
The study focuses on how S. mutans, a bacterium present in everyone’s mouth, can sometimes dominate the oral environment through complex interactions.

The study focuses on how S. mutans, a bacterium present in everyone’s mouth, can sometimes dominate the oral environment through complex interactions.
In a newly-released study led by Ryan Wyllie, Assistant Research Scientist, and Paul Jensen, Assistant Professor, Biomedical Engineering, researchers have unearthed crucial insights into the dynamics of the oral microbiome, which could significantly influence the future of dental health and shed light on how it affects a person’s overall health. The study was published in PNAS.
“As oral health goes, the identification of this system is important because it helps us understand the mechanisms by which Streptococcus mutans (S. mutans) comes to dominate the tooth surface and bring about the formation of dental caries.”
The study focuses on how S. mutans, a bacterium present in everyone’s mouth, can sometimes dominate the oral environment through complex interactions.
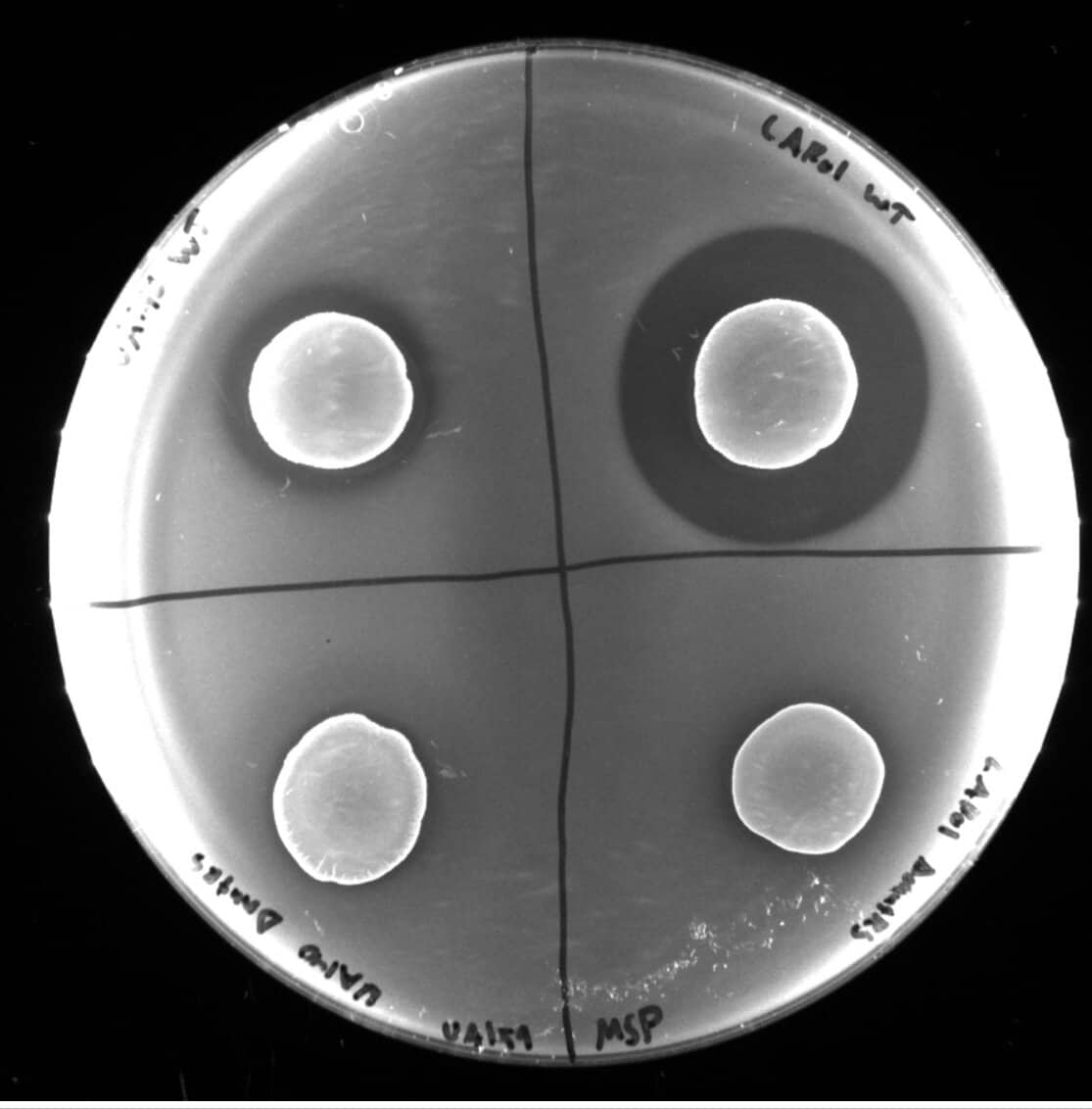
S. mutans, a ubiquitous member of the human oral microbiome, becomes a dental villain when specific ecological shifts allow it to proliferate, producing a subclass of bacteriocins called lantibiotic mutacins—broad-spectrum antimicrobials—that aggressively eliminate competition, enabling S. mutans to thrive and cause decay.
“There are a handful of bacteria that cause rapid acidification of your tooth surface when they come to dominate that microenvironment,” Dr. Wyllie said. “The buildup of acid is what ends up eroding your enamel and causing cavities.”
Dr. Wyllie noted that his team was interested in understanding the community-level changes that allow S. mutans to dominate the tooth surface. “That’s tricky because there are hundreds of different species of bacteria that make up the oral microbiome,” he added, “and they’re all interacting with one another. One of the major interaction mechanisms between the different bacterial species in the oral microbiome are small antimicrobial peptides called bacteriocins. Bacteria produce bacteriocins in an effort to kill off their ecological competition so they can acquire more resources and proliferate. The lantibiotic bacteriocins of S. mutans have been of considerable scientific interest for decades due to their broad spectrum of activity, but until now we didn’t understand the regulatory mechanisms controlling their production.”
By understanding how the production of lantibiotic mutacins is controlled, researchers can gain better insight into how S. mutans comes to dominate the tooth surface, leading to the formation of cavities.
“In our paper, we discovered that there’s actually a new class of quorum-sensing system, called MutRS, that controls the production of these antimicrobials,” Dr. Wyllie said. He explained that a quorum-sensing system is a way bacteria communicate with each other. Understanding this system opens avenues for potentially controlling the dominance of S. mutans by disrupting their quorum-sensing signals.
“Streptococci produce these tiny peptide pheromones and export them outside the cell. Nearby bacteria of the same species can then sense those pheromones and alter their behavior. So when there’s a lot of S. mutans in a particular area, the concentration of the quorum-sensing pheromone will be high, and their collective behavior will be more aggressive than it would be if there were only a few S. mutans and the concentration was low.”
The implications for oral and general health are significant. Wyllie suggests that by developing inhibitors that block quorum sensing, it might be possible to prevent S. mutans from dominating the oral microbiome, reducing cavity formation. This approach offers a promising alternative to traditional dental treatments, which often focus on treating symptoms rather than preventing underlying microbial dynamics.
“Every time you brush your teeth or choose a snack, you’re unknowingly influencing a microbial community with thousands of interactions,” Dr. Wyllie said. These daily actions affect the balance and behavior of bacterial populations, highlighting the importance of regular oral hygiene and healthy dietary choices in maintaining this delicate ecological equilibrium. Each person’s metabolic composition also plays an important role throughout the process.
Wyllie’s focus on this area began while conducting a bioinformatic survey. “Finding this system was both surprising and exciting, pointing to new frontiers in bacterial communication,” he said.
With these discoveries, the study represents a step in demystifying the oral microbiome’s complex web of interactions and provides a foundation for future research aimed at developing targeted interventions to manipulate microbial behavior to prevent and manage dental diseases.
The entire paper is available online.