A team of U-M biomedical engineers, partnered with an international team of researchers, is working to quantify vascular architecture, with the goal of using the physics of shear waves to measure and treat tumors and other medical challenges.
David Nordsletten, U-M Professor, Biomedical Engineering and Cardiac Surgery, along with Ralph Sinkus, INSERM and King’s College London, Chair and Professor, Biomedical Engineering and Research Director of CNRS/France, Paris, present a novel scattering theory, in conjunction with MRI-based elastography imaging, which enables the unraveling of a material’s innate constitutive and scattering characteristics. By overcoming a three-order-of-magnitude scale difference between wavelength and average inter-vessel distance, they demonstrate non-invasively a macroscopic measure of vascular architecture.
The physics of shear waves traveling through matter carries fundamental insights into its structure; for instance, quantifying stiffness for disease characterization. Researchers want to use the data they gather from these analyses to inform ways to target drug delivery in disease management. Their approach aims to broaden the field of imaging by using the dispersion properties of shear waves as macroscopic observable proxy for deciphering the underlying ultra-structure of vasculature.
“Mechanics is important, but how do you measure it?” asked Dr. Sinkus. “In geophysics, they are igniting dynamite to have waves that travel through the Earth, and then they look at the waves which come back on the surface, and from this they are deducing mechanics.”
Drs. Nordsletten and Sinkus explained that researchers can see waves inside an object using similar wave technology. The technology is based on seeing waves inside the object via MRI, where external vibrations are transmitted through the body to show their spatial organization as they traverse organs and tissues. From these waves, it is possible to analyze the physics.
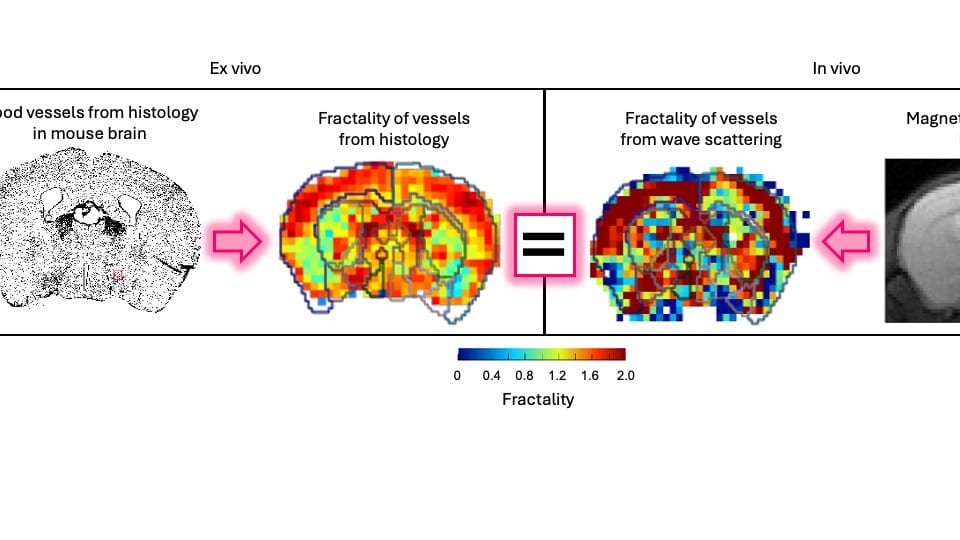
“Our dream was that these waves can tell us something about the underlying vasculature, which normally you cannot see because the vasculature is tiny,” Dr. Sinkus added. “Our image resolution is large, so you cannot see the results. Our goal is to employ our understanding of the physics of these waves propagating in heterogeneous materials to enable us to see something which we cannot see with the human eye.” With their developed theory, and evaluations in silico, in vitro, in small animals and human tissues, they showed that vascular architecture leaves distinct signatures in the propagation of waves.
“Someone is pulling the brake: Somehow this wave, while fighting through this maze of vessels, is slowed or pulled back,” Dr. Sinkus said. “This is the theory we were working on. At the microscopic scale, you don’t see these vessels because they are way too small. But the wave will probe your tissue when it travels through and it will sense it. Imagine you want to shoot a football in a forest where all the trees are aligned. It’s easy to shoot the football, but if the trees are randomly scattered, the football will go backwards, forward, backward, forward and fight its way through a maze of trees. We researched this link by how much this wave is pulled back with respect to the organization of the vessels. If the brake is pulled back, we have to ask what the cause of this pullback is.”
Dr. Giacomo Annio, the lead author of the study and a Marie Sklodowska Curie Fellow at Oslo University Hospital and Stanford Medicine, posed a thought-provoking question: “Why are vessels the key players in scattering, as opposed to other anatomical structures?” He elaborates, “Consider the brain’s intricate architecture. Many elements could be at play.” However, it is the exceptional rigidity of vessel walls’ components – particularly the smooth muscle cells, which are a thousand times stiffer than the surrounding tissue – that makes vessels the prime candidates. This striking stiffness differential holds the key to understanding their role as scatterers, ultimately unraveling the mystery.
Dr. Nordsletten continued the explanation of how these concepts serve as the foundation for ongoing research. “When you start getting tumors or cancer, there is ad hoc angiogenesis or development of vessels, and they become very unstructured and much more chaotic than what you see in native, healthy tissue,” he said. “Being able to actually measure that would be amazing to understand the structure of that tumor and potentially how effective treatments may be. Our goal focused on asking if we can actually infer something about the architecture of these vessels. But because it has a link to influencing the physics of these wave propagations, we can actually get some idea of what that would look like. The treatment becomes interesting because, for example, one of the biggest challenges with cancer treatment is the delivery of these drugs and whether or not you can get this drug to leave the vascular space, enter the tumor and actually target the cancer cells themselves, or whether these things are going to flood through the tumor without actually entering inside of it. The research idea is that this would be a novel way of providing us insight into what drugs might be appropriate for different types of tumors. It’s a pretty radical idea going from millimeters down to microns and being able to say that we can tell you something about what’s happening on a micron level. Because you’re understanding the physics of the wave propagation, and you have a theory, you’re suddenly capable of extracting that information.”