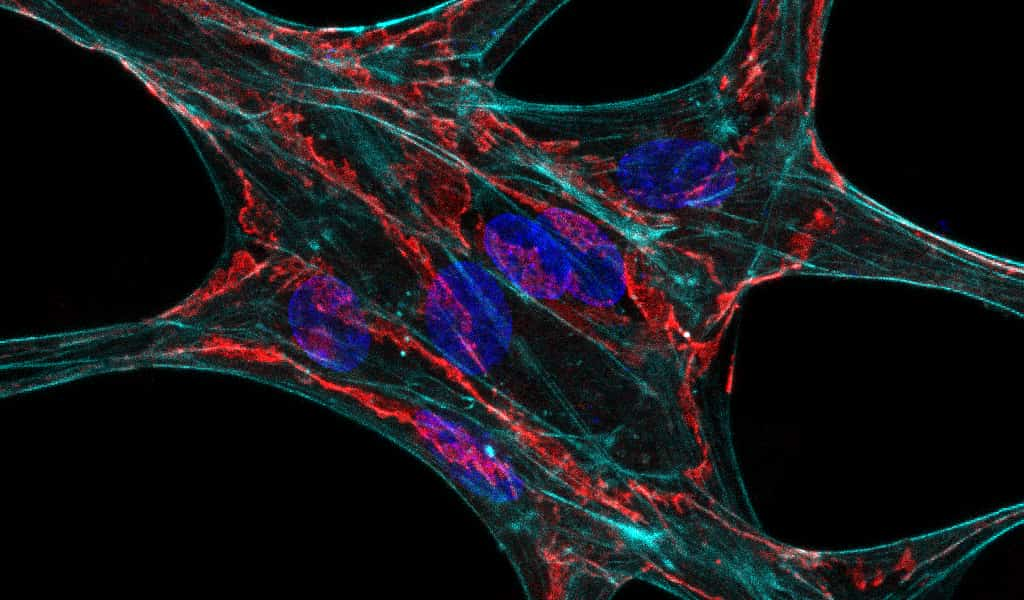
Pulling Together: Harnessing Intercellular Mechanical Forces to Build Capillaries
Engineering capillaries to form a multicellular network

Engineering capillaries to form a multicellular network
Can cells use mechanical forces as a means to communicate with each other and assemble into capillaries useful for tissue engineering and regenerative medicine? This is a question that Brendon Baker, Associate Professor in the department of Biomedical Engineering, and members of his lab are investigating, as profiled in a paper published recently in Advanced Science.
“One major strategy to engineering capillaries is to disperse individual endothelial cells (ECs) within or on top of a biomaterial and allow the cells to find each other in space, connect and form a multicellular network,” Dr. Baker said. The assembly of multicellular networks is required for the formation of capillary beds that support flow.
“While previous studies have implicated secreted factors in helping cells find each other, we were interested in whether tugging forces generated by cells are also important in this assembly process,” Dr. Baker added. His team is studying how cells transmit forces and how neighboring cells sense those forces in order to allow for intercellular communication.
“We determined the requisite material properties and then the key mechanisms that the cells use to home in on each other,” he said. “We used reductionist models to understand fundamentally what’s happening and then eventually applied our understanding to promote the assembly process in 3D biomaterials with translational potential,” Dr. Baker said.
Dr. Christopher Davidson, PhD, (U-M BME 2022) and current BME PhD graduate student Firaol Midekssa led this project. Their work, titled “Mechanical intercellular communication via matrix-borne cell force transmission during vascular network formation,” describes how cell force-mediated matrix displacements in deformable fibrous matrices underlie directional extension and migration of neighboring ECs towards each other prior, preceding formation of stable cell-cell connections. They also identified a novel role for calcium signaling and mechanosensitive ion channels during this assembly process.
Dr. Baker explained that the eventual goal of this research would be to use these vasculogenic matrices to engineer capillary beds that can improve blood flow to a patient’s tissues or organs, promote wound healing, or support the function of an engineered tissue graft.