BME researchers receive two NIH grants to focus on vision loss, prostate cancer detection
Two grants total $6M

Two grants total $6M
BME researchers have recently received funding to examine Multimodal Molecular Imaging in vision-loss detection and the clinical translation of dual-modality transrectal ultrasound and photoacoustic imaging for detection of aggressive human prostate cancer. The first project, which runs for four years, is funded by a $2.5M grant from the National Eye Institutes of NIH. The second project is funded for five years by the National Cancer Institute of NIH and is worth approximately $3.5M.
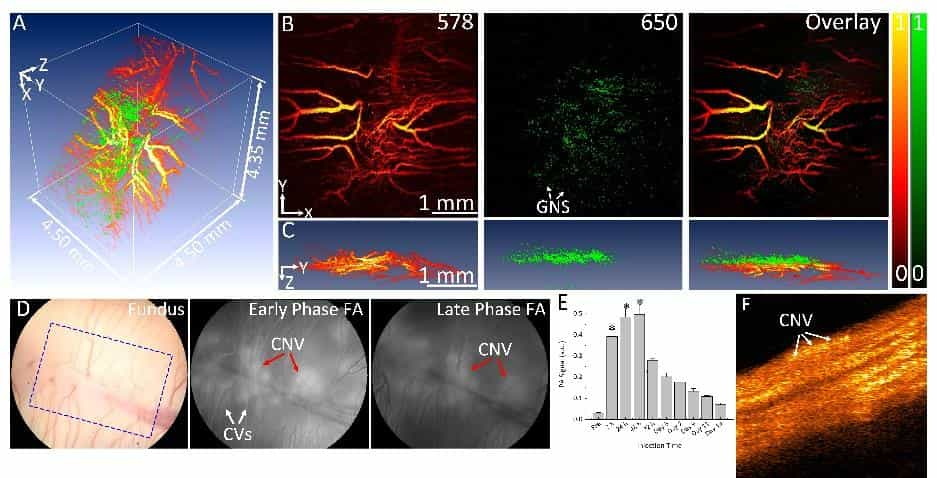
Multimodal molecular imaging of choroidal neovascularization
Wet age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the developed world. Choroidal neovascularization (CNV) is the leading cause of vision loss due to AMD. Although anti-vascular endothelial growth factor (VEGF) therapy has shown a great breakthrough in CNV treatment, persistent disease activity (PDA) is common.
U-M BME researchers have developed a high resolution, multimodal ophthalmic imaging system incorporating photoacoustic microscopy (PAM), optical coherence tomography (OCT), and fluorescence microscopy (FM). Chain-like gold nanoparticle clusters have been developed and used to enhance molecular imaging and target integrins present in CNV. The researchers have also developed a robust animal model of PDA using older rabbits that demonstrate minimal response to anti-VEGF therapy. Encouraged by these preliminary results, the research team proposes to further develop this platform molecular imaging technology for AMD with a central hypothesis that a multimodal molecular imaging system that can evaluate the CNV animal model could contribute to understanding the fundamental biology of AMD and the development of new pharmaceutical therapies to treat CNV.
While AMD is a serious problem, one critical barrier limiting the ability to test novel therapies in preclinical settings is the lack of CNV animal models with PDA and the lack of methods for longitudinal monitoring of disease biomarkers and response to therapy. The goal of this project is to develop state-of-the-art multimodal molecular imaging for non-invasive and longitudinal assessment of the imaging biomarkers in a new CNV rabbit model with PDA to offer a platform technology for the development of novel therapeutics.
The lead PIs are Yannis Mantas Paulus, MD, Associate Professor, Ophthalmology and Visual Sciences and Associate Professor, Biomedical Engineering at U-M; and Xueding Wang, the U-M BME Jonathan Rubin Collegiate Professor, Department of Biomedical Engineering and Department of Radiology, and Director of Optical Imaging Laboratory.
“AMD is a common problem in senior citizens, so this is a very big issue,” Dr. Wang said. “In order to understand this clinical condition, we have some imaging technologies already, such as OCT, which stands for optical coherence tomography. Traditionally, diseases such as AMD, which is a common problem associated with overgrowth of blood vessels called neovascularization, can only be diagnosed at a relatively late stage, often leading to irreversible damage. That’s too late. So we are working to see if some imaging technology, by detecting the molecular biomarkers of the disease, can lead to earlier diagnosis to ensure more effective treatment.”
Dr. Wang explained that this new imaging method offers great sensitivity in detecting molecular biomarkers by leveraging the contrast enhancement made possible by nano-sized gold particles. “We are able to detect the disease at a very early stage and we can see the particular molecular changes,” Dr. Wang said. “This method allows us not to rely on the tissue to change structures before we can detect the disease progression. A company called IMRA, a local company affiliated with Toyota, is building the nanoparticles in collaboration with us,” Dr. Wang said.
“BME and Ophthalmology have been working together for many years,” Dr Wang added. “We have many joint projects, and this is just one example of the successful collaboration between BME and Ophthalmology,”
Clinical translation of dual-modality transrectal ultrasound and photoacoustic imaging for detection of aggressive human prostate cancer
The second grant on which Dr. Wang is a PI is worth approximately $3.5 million over five years and focuses on the clinical translation of dual-modality transrectal ultrasound and photoacoustic imaging for the detection of aggressive human prostate cancer. The other PI of this multi-PI project is Dr. Raj Kothapalli, a professor at Pennsylvania State University.
Prostate cancer (PCa), with an annually increasing incident rate, has become the most commonly diagnosed cancer in American men. The accurate diagnosis of aggressive PCa is critical for the survival of patients. Transrectal-ultrasound-(TRUS)-guided biopsy, the current standard procedure for evaluating the presence and aggressiveness of PCa, suffers from low core yield, leading to under-sampling and under-grading of clinically significant tumors. To fill this long-standing and serious technical gap in PCa diagnosis, Dr. Wang and Dr. Kothapalli propose to develop a novel dual-modality imaging platform which integrates the emerging photoacoustic (PA) molecular imaging technique with the established TRUS for improved detection and differentiation of clinically significant PCa tumors. With the unique capability to map functional, chemical, and molecular information reflecting pathological conditions over the entire human prostate in vivo, non-invasively, the proposed TRUS and PA (namely TRUSPA) imaging can sensitively detect spatially distributed tumors and, more importantly, differentiate aggressive versus non-aggressive PCa tumors.
This proposed multi-institutional research will leverage the extensive experience of Dr. Kothapalli’s lab in developing and translating TRUSPA imaging systems for in vivo human prostate imaging, and the expertise of Dr. Wang’s lab in developing novel functional imaging biomarkers of PCa. The central hypothesis is that the TRUSPA is capable of mapping a list of functional and structural imaging biomarkers in the human prostate in a real-time non-invasive manner for detecting and differentiating clinically significant PCa.
“In a current prostate cancer clinic, the standard procedure for diagnosis is ultrasound-guided needle biopsy,” Dr. Wang said. “Under the ultrasound imaging guidance, we insert small needles to the prostate to harvest some tissues and look at them under the microscope. We need to see if there is cancer in the prostate, and if there is, we need to determine the grade of the cancer–if it’s aggressive cancer or non-aggressive cancer and how severe the condition is.”
Physicians rely on this diagnostic information to determine the next steps for treatment, such as the need to deliver chemotherapy or radiotherapy, or to perform surgery. All of these decisions depend on the diagnosis to determine the severity of the situation.
“The problem with current ultrasound imaging, which we rely on to determine where to insert the needle, is that it can be challenging to detect the cancer tumors in the prostate,” Dr. Wang added. “In current clinics, a more advanced form of imaging uses ultrasound and MRI fusion. Before having an ultrasound, the patient will receive an MRI to detect the tumors, and then during the ultrasound, the guided needle biopsy occurs, with the doctor looking at the insertion of the needles, and the MRI image is fused with ultrasound. There can still be misalignment issues, making the insertion challenging. We really need a high-powered imaging technology to guide the harvesting of tissues during the needle biopsy. In our research, we are working to build a multimodal imaging technology called photoacoustic imaging, which is laser-induced ultrasound imaging. This imaging technology can be combined with ultrasound so we can get two sets of images. The combined image can tell you a lot of information about the functional changes in the tumor. For example, cancer usually has newly formed blood vessels and sometimes has low oxygen saturation; the newly formed blood vessels are also usually leaking. The photoacoustic imaging presenting these functional changes is more sensitive to detect cancer than traditional ultrasound imaging presenting tissue structure changes, so it will significantly improve the accuracy in guiding the needle biopsy.”
The clinical study is simultaneously happening at the University of Michigan and Penn State, with each location testing 25 patients. “By researching our 50 subjects, we will test the performance of this imaging system, which will tell us whether this process can help us to detect aggressive prostate cancer,” Dr. Wang said. Dr. Wang’s team is working with the urologists, including John Wei, MD, and Simpa Salami, MD, at Michigan Medicine to recruit patients interested in participating in the study.
